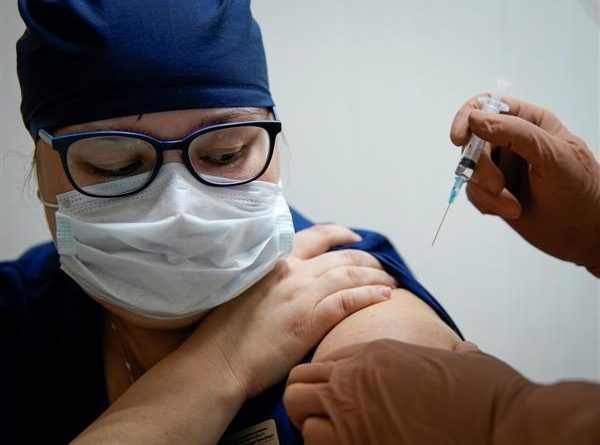
CDSCO approves Covishield, COVAXIN for restricted emergency use in India
[ad_1]
Tribune News Service
New Delhi, January 3
Central Drugs Standard Control Organisation (CDSCO) on Sunday approved Oxford-AstraZeneca Covishield and Bharat Biotech’s COVAXIN for restricted emergency use for immunisation against Covid-19 in India.
India’s COVID-19 tally of cases climbed to 1,03,23,965 with 18,177 new cases in a day, while 99,27,310 people have recuperated so far pushing the national recovery rate to 96.15 per cent on Sunday, according to the Union Health Ministry data.
There are now two vaccines on India’s pandemic management horizon with the government successfully conducting vaccine dry run at 259 sites in 116 districts pan India today. Around 1.14 lakh vaccinators have been trained and over 75 lakh beneficiaries (of first phase priority inoculation) registered on Co-WIN. Sources said once approved by the DCGI, the vaccine rollout could start as early as next week.
[ad_2]
Source link