COVID-19: Vaccine trial volunteer stresses importance of masks, handwashing
[ad_1]
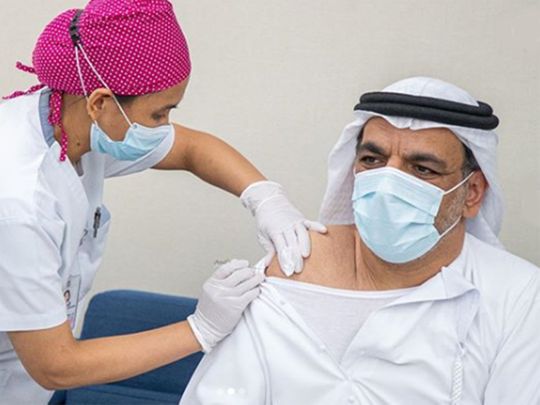
Image Credit: Courtesy: Instagram/@admediaoffice
Abu Dhabi: She may be a trial volunteer for a COVID-19 vaccine, but Hussa Al Mansoori is still diligently wearing a mask.
What’s more, she told Gulf News she was reminded to continue to follow safety measures even when she would go down to give her latest blood sample for the vaccine trials.
“I received my second vaccine dose on August 23, and I had to submit my blood sample, as well as a nasal swab for a PCR [polymerase chain reaction] test, on September 6. As I provided the sample, the workers at the trial centre [4Humanity] stressed once again that I should continue wearing a mask and must also wash my hands frequently,” said Al Mansoori, a 49-year-old Emirati IT executive based in Abu Dhabi.
“It was interesting for me because it shows the level of care we must all practise. Even if I am vaccinated against coronavirus, the instructions I received showed that we cannot just let our guard down,” she added.
Al Mansoori is one of more than 31,000 people who signed up for the vaccine trials in the UAE.
UAE vaccine trials
Sponsored by Abu Dhabi-based artificial intelligence and cloud computing firm, G42, the trials aimed to test the effectiveness and safety of an inactivated vaccine developed by Chinese pharmaceutical company, Sinopharm China National Biotec Group. The vaccine had helped volunteers successfully generate COVID-19 antibodies during the first two phases of testing in China.
Dubbed 4Humanity, the Phase III trials kicked off in the UAE on July 16, with top officials from the Department of Health (DoH), Abu Dhabi’s health-care regulator, becoming the first volunteers.
The trials, which were administered by Abu Dhabi’s public health provider, Abu Dhabi Health Services Company (Seha), were supervised by the DoH and the UAE Ministry of Health and Prevention.
The registration of volunteers in the UAE closed on August 31, but Al Mansoori said she was still receiving follow-up calls about her vaccination experience.
“The team checked with me on [Sunday morning], to ask about how I was doing, and if I am doing all right. I was happy to tell them that I was perfectly well,” she said.
Trial analysis
The Emirati volunteer said she had also asked about what results the vaccinations were yielding.
“I asked the available workers how effective the vaccines had been on my immunity to COVID-19. But I was told that the results would be analysed and revealed to me later. I don’t know when this will be, but I am really waiting to find out,” she said.
Dr Jamal Al Kaabi, undersecretary at the DoH, had earlier told Gulf News that the results of the trials are now being analysed, following the completion of 42 days, on August 26, since the first volunteers received their doses. He said that trial volunteers had received one of three types of vaccine shots — Strain 1, Strain 2 or a placebo.
Double-blinded study
As a double-blinded study, neither the researcher nor the doctor knows what treatment was administered to a particular patient. This type of research is carried out to eliminate any potential biases the researcher or volunteer may have.
“Statistical analysis will now be carried out to see any correlations between the shots and any impact they have had. It depends on the [vaccine] developer, but the aim is typically to achieve a four-fold increase in immunity from the baseline,” Dr Al Kaabi had said.
Al Mansoori said she will have to go for what she understood would be the final vaccine trial assessment on September 20, which would mark 49 days after her first vaccine shot.
Meanwhile, G42 is currently conducting trials for the inactivated vaccine for Bahrain and Jordan.
[ad_2]
Source link